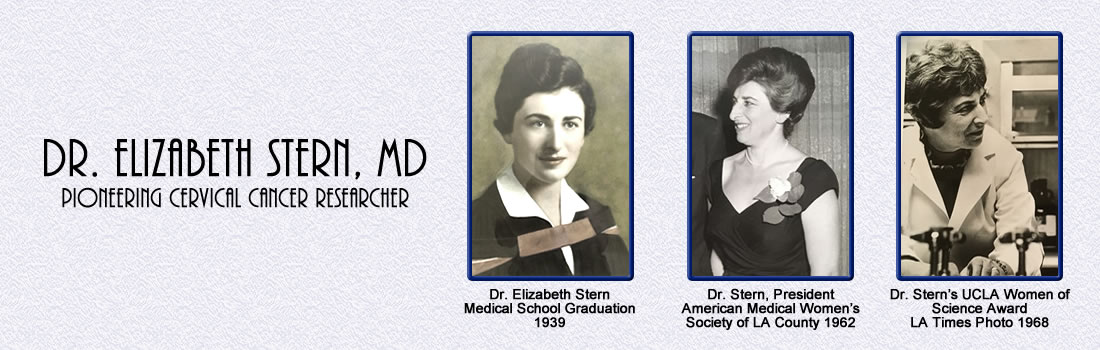
 |
 |
 |
Improved Pap Testing
Methods
In 1928, Dr. George Papanicolaou reported that analysis
of individual cervical cells, collected by a simple swab
technique could be used to detect cervical cancer.
Specifically, he showed that cancer cells displayed
changes in cell size and shape compared to healthy cells
that were visible under a microscope. In his widely
cited paper from 1941 he demonstrated that this test
could be used to detect early-stage cervical cancer
including in women that were not yet showing symptoms
[1]. This test became known at the Pap test, or Pap
smear, and has been highly effective in reducing the
rates of cervical cancer and associated mortality in the
United States [3, 4].
The Pap test helped initiate an entirely new branch of
diagnostic medicine known as cytopathology, the
diagnosis of disease by studying individual cells. The
Pap test had a major impact on women’s health. Doctors
could now identify cervical cancer by analyzing cells
collected from the surface of the cervix bypassing more
expensive and invasive tissue biopsy procedures. By the
1960s the Pap test was being implemented in private
practice and public clinics across the US and in other
countries.
However, the expanded use of the Pap test presented many
challenges. Cytopathology was still a new field, and its
development was tied to the growth of Pap testing [3].
There was a shortage of physicians and technicians
trained in analyzing cells, and experts in this area,
such as Dr. Stern, were rare. Although Pap testing was
easy to execute, diagnosing a sample as cancerous or
non-cancerous was difficult for inexperienced analysts
due to a lack of standardization in the medical
literature and problems with sample preparation.
Dr. Stern recognized these issues and dedicated her
research to improving and standardizing the detection of
cervical cancer. Dr. Stern’s work made it easier for
physicians and technicians to identify abnormal cells
and to increase the accuracy of Pap testing for cervical
cancer diagnosis [5].
Problems with the Conventional Pap Test
By the late 1970s the Pap test had been used for more
than 20 years in the United States, but few changes had
been made to the technique during this time. Yet there
were clear problems that needed to be addressed.
Many physicians were concerned by false negatives in Pap
testing as some patients with cervical cancer were
misdiagnosed as healthy. These false-negative results
were often due to improper sample collection,
processing, or analysis, with rates as high as 33% in
some laboratories [5, 6]. Of course, false positives
from the Pap test, in which healthy patients were
incorrectly diagnosed with cervical cancer, was also a
concern, and had equally high rates. This led to
unnecessary testing and anxiety on the part of patients
as well as inefficient use of hospital resources [3].
To fully understand these concerns, it is important to
know how the Pap test worked. This test is very
straightforward, making it feasible to implement in labs
worldwide.
Briefly, cells are collected from the cervix, lightly
smeared onto a glass slide, and placed in a fixative
solution that prevents cells from degrading and
preserves cell size and shape. The slides are air dried
and then stained using a series of dyes that help
distinguish cell features. Finally, Pap test slides are
analyzed by a technician or cytopathologist. The
frequency of abnormal and normal cells is noted, and
patients are followed up as needed.
This process seems simple, but each step is prone to
multiple errors that can impact the results. These are
the main problems physicians faced:
1. Cell Damage
If cells are pressed too firmly onto the glass slide,
they may burst or become distorted, thereby changing
test results. If too many cells are lost or damaged, it
can complicate analysis of a sample due to a lack of
material.
2. Immune Cells in the Sample
The cells for Pap tests are collected directly from the
cervix, but the cervix doesn’t contain just one type of
cell. Immune cells are located throughout every tissue,
constantly interacting with the environment to ward off
infections and disease. When physicians take a cervical
scraping for a Pap test, there are a large number of
immune cells in the sample that complicates the analysis
of cervical cells.
3. Cell Clumping or Overlap
Even if the cells are transferred perfectly, there may
be clumping with cells lying on top of one another,
making it impossible to analyze the shape of individual
cells.
4. Cell Counts
Early-stage cervical cancer patients have mostly healthy
cells. Abnormal, cancerous cells are rare. Thus, it is
crucial to examine as many cells as possible to
accurately diagnose patients and avoid false negative
results.
5. Long Processing Times
With the conventional Pap test, each sample had to be
individually processed, stained and analyzed by trained
cytopathology staff, requiring a considerable amount of
hands-on time. The lack of knowledgeable staff along
with the increasing number of samples4 contributed to
long processing times and placed additional stress on
overworked cytopathology staff.
6. Human Error
The above problems, combined with inexperienced
cytopathology staff and poorly defined stages of
cervical cancer, led to Pap testing results with high
false negative and false positive rates [3, 5, 6]. Dr. Stern had been working in the fields of
cytopathology and cervical cancer since the late 1940s
and was well aware of these concerns from running Pap
testing clinics in Los Angeles County.
In an effort to standardize testing results and provide
guidance for physicians, she designed and validated a
100-point scale for scoring cervical cell abnormalities
in Pap tests and biopsies [7]. This scale ranged from
healthy to invasive cancer and included very early
changes in cell size and shape that were difficult for
non-cytopathologists to identify.
However, this scaling system did not solve all the
problems associated with the conventional Pap test. Cell
damage was still a concern, as was removing immune cells
and other contaminating cell types. New methods were
needed to make samples cleaner, accelerate Pap test
analysis, and make the results more reliable.
Collaboration with JPL to Improve Pap Testing
In the mid 1970s, Dr. Stern and her colleagues at UCLA,
Claire McLatchie and Dr. Dorothy Rosenthal, connected
with scientists at the Jet Propulsion Laboratory (JPL)
to begin working on an automated Pap test.
Dr. Stern and colleagues were interested in providing
clinicians with a digital analysis that would ease the
burden of conventional hands-on analysis on
cytopathology staff. Although the role of
cytotechnicians and cytopathologists could not be
completely eliminated, they reasoned it would be helpful
to flag abnormal cell samples for additional hands-on
analysis, while the remaining healthy samples, which
comprised the majority, could be marked as normal.
The scientists at JPL, Drs. Kenneth Castleman and
Benjamin White, were experts in microscopy and digital
imaging analysis. Their long-term objective was to
develop a reliable, automated system using microscopic
analysis of individual cells to detect disease. They
based this digital processing system on Dr. Stern’s
100-point scale with the goal of developing a computer
program that labeled cells in the same way as expert
cytopathologists or cytotechnicians. The researchers
devised a two-stage plan as follows:
1. Developing cell processing techniques to remove
non-cervical cells and disperse cervical cells into a
single layer on slides. This process had to be gentle
enough to preserve cell shape and size but firm enough
to break up cell clumps.
2. Working with JPL to develop an automated, digital
measuring system. Cells that represented different
categories along Dr. Stern’s cervical cancer scale would
be measured by JPL’s system. These digital measurements
would be compared with the conventional hands-on
measurements to identify distinct stages of cancer.
Optimizing Cell Processing Methods to Improve Pap
Testing
The first step in this project was to develop a
technique that made individual cervical cells visible
for automated measurements. Ideally, this method would
also remove immune cells and mucus.
Dr. Stern reviewed contemporary work on cell preparation
for cytopathology analysis [2, 8, 9] and adopted newer
strategies. The main differences from the conventional
Pap test described above are:
• Instead of smearing cells directly onto a glass slide,
the swab or brush containing the cervical sample is
placed in a vial containing a fixative solution.
• To enrich cervical cells, the sample is strained
across a nylon mesh filter capturing large cervical
cells, while smaller immune cells and mucus flow through
the filter and are discarded.
• To break up cell clumps, captured cervical cells are
gently mixed using a syringe.
• Finally, cells are applied to another filter and
transferred to a glass slide for Pap staining and
analysis.
The difference between the two methods can be visualized
by looking at slides.
Figure 1A shows a Pap test prepared using the
conventional method. The red box shows small immune
cells and the purple box shows a clump of cervical
cells.
Figure 1B shows a Pap test prepared using Dr. Stern’s
new technique. There are very few infiltrating immune
cells, and the cervical cells are separated and easily
visualized. Dr. Stern’s group found that 92% of immune
cells were removed from the sample using their
procedure, and only 2% of cervical cells were lost over
the filter [2].
Dr. Stern’s new technique was a major scientific advance
taking methods that had been applied to other cell types
and using them to develop a technique that allowed
doctors to more easily analyze cervical cells.
Automated Digital Image Analysis of Pap Tests
With this method in hand, Dr. Stern and her colleagues
went to JPL to develop a digital imaging system [10,
11]. Although automated Pap tests were not unheard of at
the time, none had been successful.
To validate their system, Dr. Stern’s lab analyzed 7,000
individual cervical cells, both manually and using the
computer system developed by JPL [11]. Cells were
derived from 105 normal and 96 abnormal cervical
scrapings with each sample prepared using Dr. Stern’s
technique.
The cells were assigned to categories along Dr. Stern’s
scale during the manual analysis. They were then
compared to the digital measurements both within and
across cell categories. The results were very promising.
In fact, the computer system could detect more small
changes and rank samples at a more precise level than
the original hands-on cellular scale [11]. These data
supported the additional development of automated and
computerized Pap test systems.
Long-term Impact and Conclusions
The most important contribution of Dr. Stern’s research
was the new cell processing technique, not Pap testing
or digital analysis. This technique helped establish
an entirely new version of the Pap test at the
cutting-edge of modern cytology, now known as the
Liquid-Based Pap test.
The major improvements in this technique are as follows:
• Cells did not have to be immediately smeared onto a
glass slide. Instead, doctors could simply collect the
cervical cells using a swab or brush and place them in a
vial.
• The capped vials were more easily transferred from
clinic to lab than glass slides and could be stored for
longer periods of time prior to processing and analysis.
• By removing cell clumps, mucus and non-cervical cells,
samples were much easier to analyze. This allowed
physicians to more efficiently and accurately label
samples as abnormal vs. normal and improved turnaround
times.
Other researchers took notice. The coming decades would
see advances making this process faster and more
consistent across laboratories. The first Liquid-Based
Pap test, ThinPrep, was FDA approved in 1996, and used a
technique similar to Dr. Stern’s.
Liquid-Based Pap tests are now the gold standard of Pap
testing in the United States and around the world. In
addition to the advantages listed, these Liquid-Based
Pap tests can be combined with testing for Human
Papillomavirus (HPV), one of the leading causes of
cervical cancer [12]. Dr. Stern’s work helped pave the
way to these modern cytopathology technologies,
transforming cancer diagnostics and saving countless
lives.
Footnotes:
Although the Pap test is named for Dr. Papanicolaou,
it was first presented by Romanian physician Dr. Aurel
A. Babes in 1927, a year before Dr. Papanicolaou
presented his own work in 1928. Dr. Babes did not
continue working on this test and only published a
single paper. Thus, the test was ultimately named for
Dr. Papanicolaou, who continued working on the test and
promoted its use as a screening tool (see References:
[3, 4]).
The American Cancer Society played an important role
by promoting
promoted the use of the Pap Test in the
early 1960s.
Pap tests are analyzed by trained cytotechnicians
and/or cytopathologists. A cytotechnician may also be
referred to as a technician or a cytologist and has a
bachelor’s or master’s degree. A cytopathologist is a
physician who has performed their residency in pathology
and focused on the diagnosis of disease by tissue or
cell analysis. The terms physician, doctor, and
cytopathologist are used throughout the text. From the
1940s through the 1960s cytotechnicians or
cytopathologists may not have been accessible for Pap
test analysis depending on location in the US. This
resulted in medical personel without special training
analyzing Pap test slides.
The accuracy of conventional vs. liquid-based Pap
tests is still debated by some experts [13].
Nevertheless, the liquid-based test has allowed for more
standardized testing and currently accounts for 90% of
Pap tests in the US [5].
In the 1970s, to help overcome inconsistencies in Pap
testing results, the American Cancer Society recommended
women have a Pap test once a year. If a cancer diagnosis
was missed by an inaccurate test, it would likely be
detected the following year. Because cervical cancer
often develops slowly over the course of at least ten
years, detecting cancer a year late would usually not
result in a serious problem. With the advent of improved
Pap tests, the American Cancer Society now recommends
women have a Pap test performed every three years.
Dr. Dorothy Rosenthal was interviewed as part of
Elliott’s Scientific American piece on Dr. Stern and
provided valuable insight on the impact of Dr. Stern’s
work on the Pap test with JPL as well as the challenges
associated with working in a new field, cytopathology.
Dr. Rosenthal was interviewed in 2017.
Despite multiple efforts by researchers and companies,
automated Pap test analysis was not commercially
successful. This was due to complications in
standardizing imaging analysis, as well as significant
up-front costs. According to Dr. Rosenthal, if a system
was both prone to error and expensive, hospitals were
not interested.
The term ‘liquid-based’ refers to the fact that cells
are taken out of a solution and placed onto a slide
rather than smeared directly onto the slide.
References:
1.
Papanicolaou, G.N. and H.F. Traut, The diagnostic
value of vaginal smears in carcinoma of the uterus.
1941. Arch Pathol Lab Med, 1997. 121(3): p. 211-24.
2.
Rosenthal, D.L., et al., A simple method of producing
a monolayer of cervical cells for digital image
processing. Anal Quant Cytol, 1979. 1(2): p. 84-8.
3.
Tambouret, R.H., The evolution of the Papanicolaou
smear. Clin Obstet Gynecol, 2013. 56(1): p. 3-9.
4.
Shaw, P.A., The History of Cervical Screening I: The
Pap. Test. Journal SOGC, 2000. 22(2): p. 110-114.
5.
Gibb, R.K. and M.G. Martens, The impact of
liquid-based cytology in decreasing the incidence of
cervical cancer. Rev Obstet Gynecol, 2011. 4(Suppl 1):
p. S2-S11.
6.
Nanda, K., et al., Accuracy of the Papanicolaou test
in screening for and follow-up of cervical cytologic
abnormalities: a systematic review. Ann Intern Med,
2000. 132(10): p. 810-9.
7.
Stern, E., et al., A cytological scale for cervical
carcinogenesis. Cancer Res, 1974. 34(9): p. 2358-61.
8.
Barrett, D.L. and E.B. King, Comparison of cellular
recovery rates and morphologic detail obtained using
membrane filter and cytocentrifuge techniques. Acta
Cytol, 1976. 20(2): p. 174-80.
9.
Husain, O.A., B.A. Page-Roberts, and J.A. Millet, A
sample preparation for automated cervical cancer
screening. Acta Cytol, 1978. 22(1): p. 15-21.
10.
Rosenthal, D.L., et al., Endocervical columnar cell
atypia coincident with cervical neoplasia characterized
by digital image analysis. Acta Cytol, 1982. 26(2): p.
115-20.
11.
Stern, E., et al., An expanded cervical cell
classification system validated by automated
measurements. Anal Quant Cytol, 1982. 4(2): p. 110-4.
12.
Sherman, M.E., et al., Cervical specimens collected
in liquid buffer are suitable for both cytologic
screening and ancillary human papillomavirus testing.
Cancer, 1997. 81(2): p. 89-97.
13.
Davey, E., et al., Effect of study design and
quality on unsatisfactory rates, cytology
classifications, and accuracy in liquid-based versus
conventional cervical cytology: a systematic review.
Lancet, 2006. 367(9505): p. 122-32.
|
|
|
|
|
|
|

